GMP Annex 1 – Selection Criteria for Protective Cleanroom Garments
By Steve Marnach from DuPont.
For the first time since 2008, Annex 1 of the EU Good Manufacturing Practice (GMP) Guideline for the manufacturing of sterile medicinal products has undergone a profound revision. The new GMP Annex 1 was published on 25 August 2022 and it is more than a simple update. The actual guideline has been completely rewritten and not only has the length of Annex 1 been increased from 16 to 58 pages, but the whole approach described therein has changed. This will have repercussions on both the technologies and the procedures used in pharmaceutical manufacturing.
The following excerpt from Section 2.2 on page 4 summarizes the new approach:
“Processes, equipment, facilities and manufacturing activities should be managed in accordance with QRM (Quality Risk Management) principles that provide a proactive means of identifying, scientifically evaluating and controlling potential risks to quality. (…) Monitoring or testing alone does not give assurance of sterility.”
It is expected that all activities inside pharmaceutical manufacturing will be governed holistically by QRM principles and documented in the contamination control strategy (CCS). It is further expected that the CCS will be a living document, based on a data-driven scientific approach, which should be continuously updated and improved upon in order to control potential risks to quality. The new GMP Annex 1 is calling for a proactive approach and simply reacting to and correcting detected contamination will no longer be enough. It is expected that manufacturers will fully understand their processes and procedures, such that they can identify the potential risks to quality, put in place all the technical and procedural means necessary to control these risks, and aim for continuous improvements. Since cleanroom garment systems are a critical part of sterile and aseptic manufacturing, they too need to be managed under QRM principles.
QRM Principles for Cleanroom Garments
QRM starts with a complete data-based analysis and understanding of all the risks to quality linked with cleanroom operators wearing cleanroom garments. Such a review will allow the analyst to design certification, qualification, validation, and monitoring procedures which have quality built into them, forming part of a holistic CCS. A risk analysis is needed to understand the contamination risks coming from operators wearing cleanroom garments. Operators represent the biggest source of contamination inside cleanrooms, resulting in 75% of all contaminants.1,2 This contamination is coming from both the operators themselves and from their cleanroom garments.
The ‘human’ contamination coming from operators is due both to biology (an average person sheds 40,000 particles per minute and 10% of those carry micro-organisms) and behaviour. While it is possible to mitigate the latter through careful operator selection, training, slow movement, and impeccable hygiene, the fact is (as multiple studies have proven) that operators will always shed particles. The only measure to prevent particles generated by operators from contaminating the cleanroom is the wearing of cleanroom garments, which are the only barrier between the operator and the production environment. The 2022 GMP Annex 1 clearly points this out in Section 7.13 i.
"The protective clothing should minimize shedding of fibres or particles and retain particles shed by the body."
As noted above, cleanroom garments themselves may be a source of contamination and this risk also needs to be assessed. For example, the material used for making the garments (non-woven for single-use garments or woven for reusable items) can shed a greater or lesser number of particles depending on the nature of the fibers or filaments used, their resistance to abrasion or their construction. The trims (zippers, buttons, elastics or sewing threads) may also be a source of contamination, while the design of the garment can also play a role, and these should also be evaluated. One detail which is often neglected is the packaging in which the cleanroom garments arrive, which could also be a source of contamination (i.e. paper bags versus plastic bags).
Main Stages of Validation
Once risks have been evaluated, the causes of those risks should be removed or replaced by technical or organisational means in so far as is possible. Residual risks should be mitigated as far as possible using a validated cleanroom garment system. The EU General Guidance on Validation (GMP Annex 1519) provides a general framework which can be applied to the qualification of cleanroom garment systems. This validation approach consists of five steps: the definition of a user requirements specification (URS), a design qualification (DQ), an installation qualification (IQ), an operational qualification (OQ), and a performance qualification (PQ). While the DQ and IQ have the highest impact on the quality achieved, the other stages should not be neglected and it is important to proceed step-by-step.3
User Requirements Specification (URS)
While they are not formally part of the validation process, it is important to define upfront the requirements on a cleanroom garment system from the user and the environment they work in. The URS will define the critical requirements against which the garment system needs to be assessed such that it will be in line with the risk assessment. For example, a trained operator may have to be able to work for at least 3 hours in the same set of cleanroom garments without causing unacceptable levels of contamination of the garments and the aseptic working environment (according to current GMP (cGMP)). The packaging system of the garment may have to be suitable for the layout of the cleanroom and its material pass-through system, or may have to be suitable for manual spray disinfection. Or, the operator may sometimes need chemical or biological protection against the substances they are handling in the cleanroom.
Design Qualification (DQ)
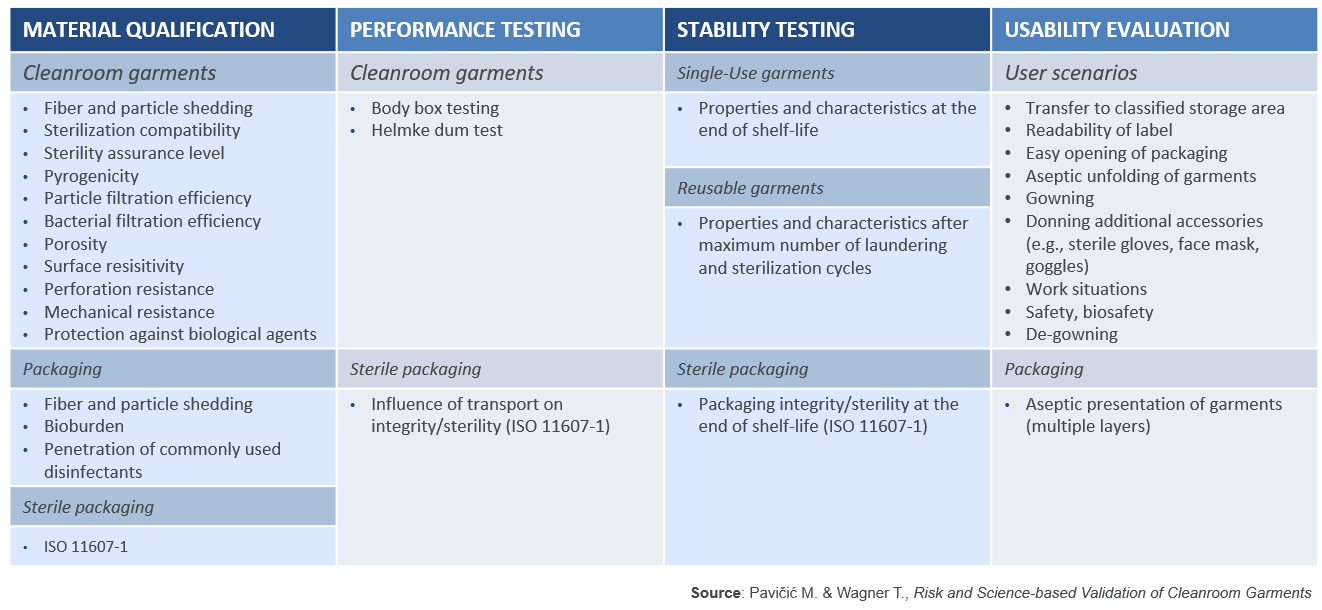
The compliance of the cleanroom garment system with cGMP must be demonstrated and documented during the DQ, in order to confirm that the selected cleanroom garment is qualified for the intended use. As the new Annex 1 will require a data-driven scientific approach, the DQ should include tests to simulate the intended use and the performance of the garment. As recommended by ISO 11607-1, the DQ should be split into four key areas: material qualification, performance testing, stability testing, and a usability evaluation. For reusable garments this needs to be extended to the garment maker’s subcontractors, suppliers, and service providers. PAVIČIĆ and WAGNER have provided a table listing the properties which should be assessed (Table 1).3
Table 1: Risk and Science-Based Validation of Cleanroom Garments
Extrusion-based bioprinters use pressure to drive the sample though a nozzle and create a pre-defined shape. Because the pressure can easily be adjusted for different viscosity sample types, extrusion-based bioprinting is often favored by researchers for its flexibility. Bioprinters based on the use of electric fields offer the highest precision. However, this comes at a price and methods must be rigorously optimized to avoid causing cellular damage.
In this article, only a couple of these properties will be highlighted to show their importance, together with the scientific test methods which may be used to assess the performance of the cleanroom garment system.
Material Qualification
In order to check whether a garment is truly sterile it is important to check if the manufacturer is following a validated sterilization process, can guarantee a sterility assurance level (SAL) of 10-6 (as per ANSI/AAMI/ISO 11137-1), and is able to document this in a certificate of sterility. A simple certificate of irradiation or a document attesting to an internal autoclaving process are not enough.
Since cleanroom garments need to be a barrier against ‘human’ contamination generated by operators, it is important to assess the filtration efficiency of the materials used in making those garments (e.g. non-woven or reusable polyester fabrics). Particle filtration efficiency (PFE) against dry particles can be assessed with test method EN 143 (TSI 8130), which measures filtration efficiency using 0.3µm diameter salt particles. Bacterial filtration efficiency (BFE) can be assessed with test method ASTM F2101.
Performance Testing
The Helmke drum test (IEST-RP-C003.4) is a good way to assess the particle shedding of cleanroom garments, especially for garments that are washed multiple times. Meanwhile, the body box test (IEST-RP-CC003.4) is the only test method to assess particle shedding when a garment is being worn by an operator. We can thus evaluate both the particle shedding of the garment and its PFE and BFE with respect to particles shed by the operator.
Stability Testing
It is important to check how the characteristics and properties of a garment will change over time due to aging, wear, and wash–dry sterilization cycles. Therefore, the performance metrics listed above should be validated under worst-case conditions. For example, for single-use items, by assessing garments from different batches and at the end of their shelf-life; while, for reusable garments, by assessing after 10, 20, 30, 40, and 50 wash–dry sterilization cycles to determine their end-of life performance. Studies by ROMANO, LJUNGQVIST, and REINMÜLLER have demonstrated that repeated washing deteriorates garment performance.4,5

Usability Evaluation
It is important to go through user scenarios and to assess the packaging of cleanroom garments to ensure that they can be used with acceptable remaining contamination and safety risks. While this is typically done by the end-user, suppliers can also perform evaluations and supply data to users.
Installation Qualification (IQ)
Even though the IQ is a formal check to verify if all required elements of the cleanroom gowning system are present, it is important to check the following in order to eliminate unforeseen risks:
- Are the gowning and de-gowning facilities in order?
- Did the supplier provide the required certificates of conformity and/or analysis, supplier instructions, etc?
- Have the standard operating procedures (SOPs) for gowning and de-gowning been written or adapted?
- Have the logistical processes for garments and accessories been validated?
- Have the operator training and qualification plans been established?
Operational Qualification (OQ)
The OQ aims at qualifying the gowning and de-gowning concept, including logistics and material pass-through, as well as the aseptic presentation of the garments (i.e. folding and packaging).
Performance Qualification (PQ)
The PQ is typically completed under worst-case conditions, which must be determined based on a risk assessment in order to validate the performance of the cleanroom garment system when it is used. The requirements specified in the URS must be complied with fully. They include an aseptic gowning qualification and validation of the microbiological quality of gowned personnel, with garments and other accessories, during actual work. Of course, it does not end there: periodic revalidation of the garment system, constant monitoring, and critical review of changes to the garment or the system are important in order to demonstrate the state of control.
Conclusion
Cleanroom garment systems are a critical part of a CCS and of process validation. A risk- and science-based, quality-by-design approach with verification is the correct strategy for controlling contamination risks related to people and offers designed-in risk reductions. This approach is an adequate response to the latest regulatory requirements.
References
[1] Ramstorp M (2000) Introduction to contamination control and cleanroom technology. Wiley VCH, Weinheim, Germany.
[2] Ramstorp M (2019) Cleanroom garments from a quality risk management approach. Eur J Parenter Pharm 24(3):4–16.
[3] Pavičić M, Wagner T (2019) Risk & science-based validation of cleanroom garments. IVT Network. https://www.ivtnetwork.com/article/risk-science-based-validation-cleanroom-garments
[4] Ljungqvist B, Reinmüller B (2005) Aseptic production, gowning systems and airborne contaminants. Pharm Technol Suppl(2): https://cdn.sanity.io/files/0vv8moc6/pharmtech/75a6ab7e927edd600f5286a1eabb2b41171af658.pdf/article-160408.pdf
[5] Romano F, Ljungqvist B, Reinmuller B et al. (2016) Performance test of technical cleanroom clothing systems. Proceedings of Indoor Air 2016, 14th International Conference on Indoor Air Quality and Climate, Ghent, Belgium.





